Back Titration Method
Heat the solution to boil to remove dissolved carbon dioxide. In this method an excess of a standard solution of EDTA is added to the metal solution being determined so as to complex all the metal ions present in the solution.
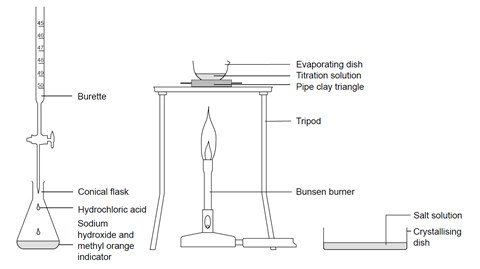
Titrating Sodium Hydroxide With Hydrochloric Acid Experiment Rsc Education
This method becomes necessary if.

. A standard solution of a reagent of known concentration is called the titrant or titrator. Save Exit. To figure out the amount of titrand in the solution a known amount of a different chemical is added to the titrands solution.
An ample supply of QC sample material shall be available for the intended period of use and shall be homogeneous and stable under the anticipated storage. Or if access becomes slow or incomplete due to system back-up procedures Internet traffic volume upgrades overload of requests to servers general network failures or delays or any other cause that may from time to time make the. In acidic solution it undergoes a redox reaction with ethanedioate ions C 2 O 4 2-The MnO 4- ions are reduced to Mn 2 and the C 2 O 4 2-ions are oxidised to CO 2.
Obviosuly it is important only when transferring sample titrant or stoichiometric reagents used for back titration. This chemical is called the titrand. Your doctor may prefer a 2 night sleep study with.
Repeat titration and boiling till yellow color doesnt return after cooling the solution. Potassium manganateVII KMnO 4 is a deeply coloured purple crystalline solidIt is a powerful oxidising agent. Titration is a method commonly used in chemistry to figure out the amount of a chemical in a solution.
The chemical of unknown concentration is called the analyte or titrand. That is a user needs to find the concentration of a reactant of a given unknown concentration by reacting it with an excess volume of another reactant of. Background Argentometric Titrations In order for a titrimetric method to be viable the titration reaction 1 must be complete ie Ktitration is.
Department of Environmental Protection-approved SOP get the pdf file for lakes or for rivers Background Information pH is a measure of the hydrogen ion concentration of the water as. Generates a titration curve from which the endpoint is determined. Solution may change color back to yellow.
Note that this method requires a 0025 mol L1 EDTA solution while the total Ca2 and Mg2 method requires a 005 mol L1 EDTA solution. Investigate and take corrective action to bring the test back into control before proceeding. The more I realised it was the right quitting method for me.
Overview of essential cookies. ARM Project Home Info for Volunteers Results pH and Alkalinity Lake Sampling Method - River Sampling Method - Analysis Protocols - pH Electrode Maintenance For the Mass. For the potentiometric method an automatic titrator will be used to perform the titration and to obtain the titration curve.
Titrate with HCl solution till the first color change. The Winkler test is used to determine the concentration of dissolved oxygen in water samples. Back Cookies used on this site.
Titration also known as titrimetry and volumetric analysis is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte a substance to be analyzed. While this is also intrinsic characteristic of the method it can be adjusted for by blind trials. We also need some glassware and apparatus to carry out the titration.
Nicola is feeling the benefits of quitting smoking after the pandemic made her think more seriously about her health and wellbeing. What is Back Titration It is basically an analytical technique in chemistry which is performed backwards in the method. According to the reaction equation.
A reagent termed the titrant or titrator is prepared as a standard solution of known concentration and volume. Summary of Test Method. Small errors in amounts of other substances.
Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration Version. HCl NaOH NaCl H 2 O. This chemical called the titrant or titrating solution reacts with.
Dissolved oxygen DO is widely used in water quality studies and routine operation of water reclamation facilities to analyze its level of oxygen saturation. 2MnO 4-aq 16H aq 5C 2 O 4 2-aq 2Mn 2 aq 8H 2 Ol. Titration is also known as titrimetry or volumetric analysis.
The remaining excess reagent is then titrated with another second reagent. The volume of titrant that is reacted usually to produce a color change is called the titration volume. When sleep apnea is diagnosed during the overnight sleep study a titration is then performed to determine the optimal CPAP pressure setting required to resolve apnea episodes.
This would be called a split night study. Then there are errors that can be connected with volumetric glass accuracy. In the test an excess of manganeseII salt iodide I and hydroxide OH ions are added to a water sample.
The excess of EDTA left after the complex formation with the metal is back titrated with a standard solution of a second metal ion. Of Ca2 in a sample may be used with the method for measuring total Ca2 and Mg2 concentration using Eriochrome Black T indicator to determine by difference the concentration of Mg2 ions in the sample. E203 Test Method for Water Using Volumetric Karl Fischer Titration.
10mL 20mL 25mL pipettes are common. Sometimes the titration is performed during the second half of the overnight sleep study. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent.
We use a pipette to measure out a known volume of the sample solutionThis is a much more accurate measurement than if we used a measuring cylinderA pipette is a glass tube with a fixed volume pipettes are available in a range of volumes eg. The titrant reacts with a solution of analyte which may also. A brief note about analytical cookies.

Back Titration Example Youtube
Difference Between Back Titration And Direct Titration Definition Examples Applications

No comments for "Back Titration Method"
Post a Comment